High Quality CBD

Como é que produzimos o nosso óleo CBD?
A criação de óleo e produtos de CBD de alta qualidade, como os da Enecta, requer competências, procedimentos e equipamento adequados ao objetivo. Nada é deixado ao acaso. Como sabemos que é essencial comprar e consumir com total tranquilidade, parece-nos correto explicar o percurso que leva uma semente de Cannabis Sativa L. a transformar-se em extrato de CBD, pronto para consumo.
A criação de um produto de alta qualidade requer a adesão a alguns princípios básicos, princípios esses que, por vezes, estão incluídos em directrizes criadas por organismos independentes.
Os princípios fundamentais que orientam a nossa produção são:
- Cultivo em campos seleccionados sem utilização de pesticidas
- Aplicação das directrizes relativas às boas práticas agrícolas e de recolha (BPAA)
- Controlo de contaminantes de acordo com as especificações da Farmacopeia Europeia
- Boas práticas de fabrico
As directrizes GACP e GMP funcionam como base para o estabelecimento de um sistema de Garantia de Qualidade adequado, fornecendo princípios úteis para garantir normas fixas, assegurando:


- O cumprimento dos requisitos gerais e específicos de higiene (que incluem a contaminação/contaminação cruzada, a higiene pessoal e a produção sanitária de material vegetal).
- Métodos de controlo
- Procedimentos documentados (SOP) que abrangem todo o processo de produção em pormenor
- Segurança do processo
- Adequação do produto final.

Cumprir as directrizes GACP e GMP: as plantas de canábis enectas garantem a elevada qualidade dos produtos CBD, como o óleo CBD. Esta política ajuda-nos a garantir, através de procedimentos normalizados e da identificação das fases críticas de produção (com base nos procedimentos HACCP, Análise de Perigos e Pontos Críticos de Controlo), que a qualidade e a segurança dos nossos produtos são adequadas e consistentes, no âmbito de um sistema de rastreio interno completo e atualizado (cada lote de produção é rastreável e identificável para o produtor e, por conseguinte, para o campo e local exato onde as plantas foram cultivadas e colhidas).
Os nossos produtos (para além de seguirem as directrizes do GACP) são certificados: Made in Italy, sem pesticidas, sem metais pesados (garantimos a ausência de metais pesados através de análises e amostragens durante as fases de produção) e armazém monitorizado (os nossos edifícios são constantemente limpos, ventilados e controlados, com áreas de trabalho específicas, bem delimitadas e adequadas para proteger os produtos de qualquer tipo de contaminação potencial).
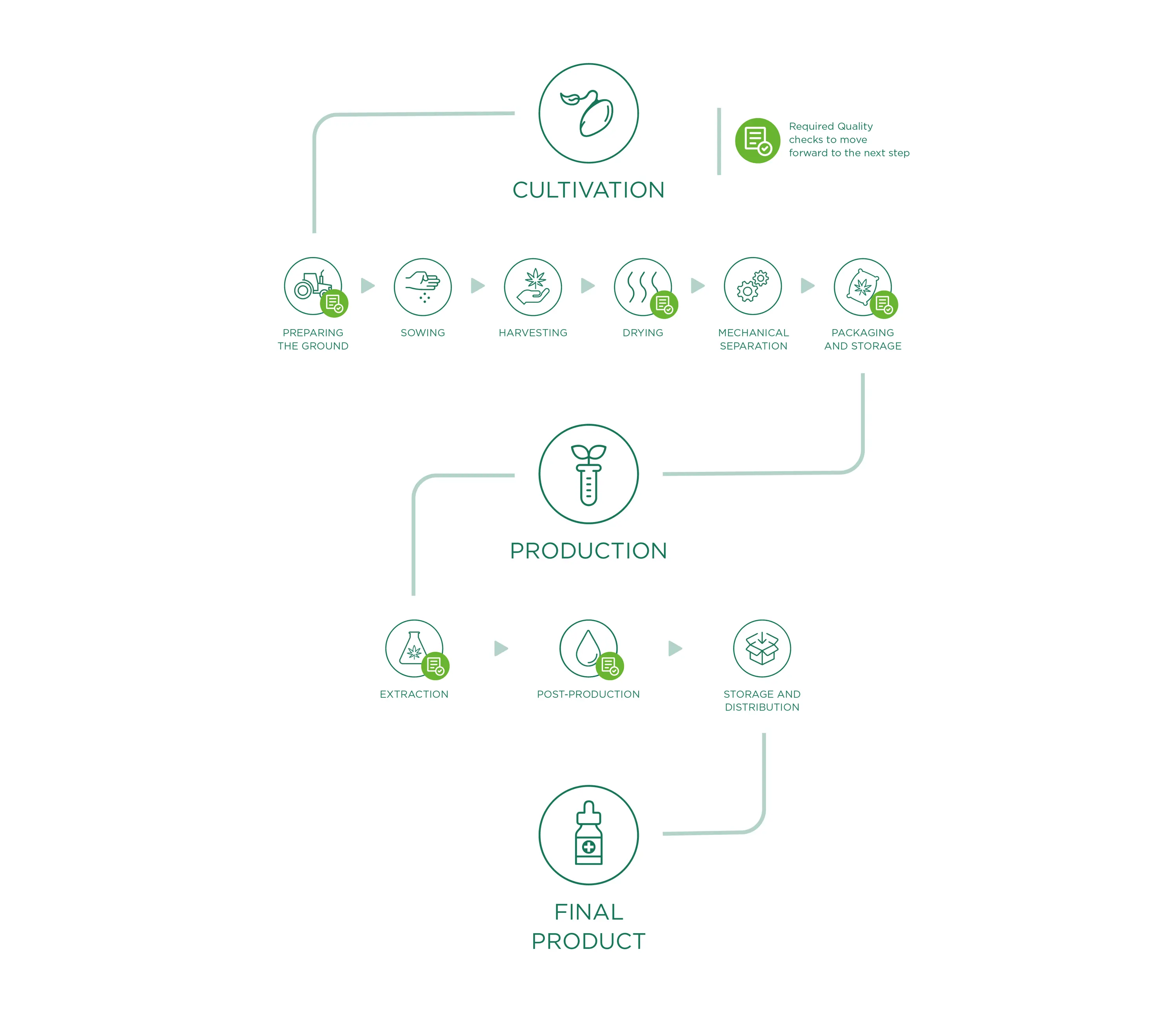
Cultivo
O material de partida para todos os produtos Enecta é a Cannabis Sativa L. Esta planta extraordinária tem muitas variedades diferentes, cada variedade (também chamada "estirpe") tem características peculiares. Desde 2013 que a Enecta investe num programa de investigação genética de sementes para desenvolver plantas de cânhamo especificamente adequadas à produção de canabinóides. Para garantir um óleo CBD perfeito, certificamo-nos de que a semente de Cannabis correcta é semeada, esta é talvez a decisão mais importante que temos de tomar todos os anos.
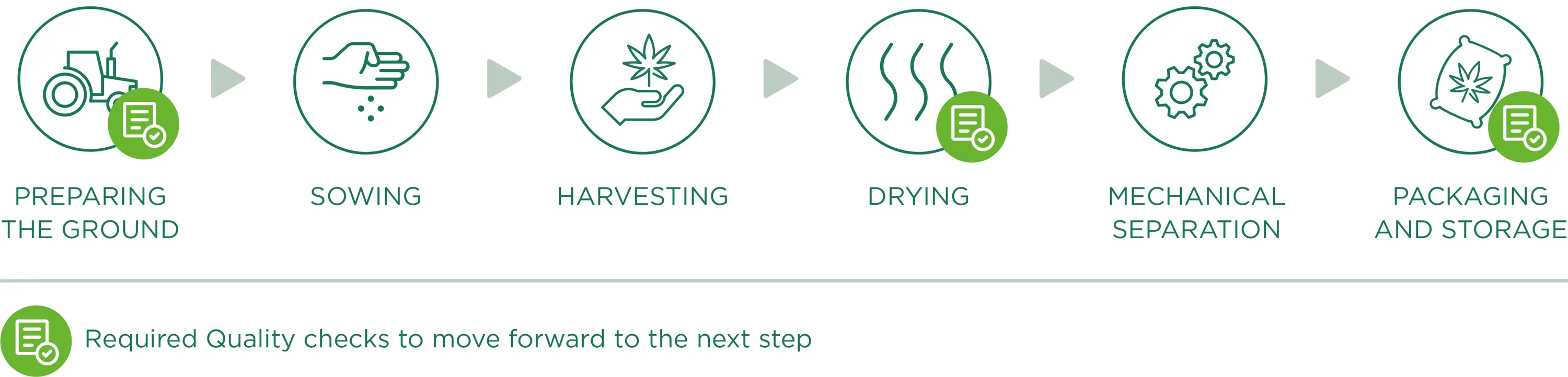
Preparar o terreno
A preparação do terreno para a sementeira é o ponto de partida e um dos factores fundamentais da fase de produção: um bom campo (em termos de composição, nutrientes e contaminantes) será efetivamente capaz de produzir um bom produto. Por isso, trabalhamos para criar um solo "hospitaleiro" que se torne o habitat natural das raízes, criando condições óptimas de crescimento com um controlo cuidadoso e preciso para evitar o crescimento de ervas daninhas ou a presença de contaminantes.
Semeadura
Plantamos apenas variedades industriais registadas no Catálogo Europeu de Variedades de Espécies de Plantas Agrícolas, em conformidade com a Diretiva 2002/53/CE do Conselho Europeu de 13 de junho de 2002, art. 17, com um nível de THC inferior a 0,2% (sementes corretamente identificadas e certificadas). Todas as informações relativas aos lotes de terra dedicados à cultura do cânhamo são registadas e documentadas, desde a sua localização até às condições de crescimento das plantas, para um acompanhamento contínuo do crescimento da cultura.
Sublinhamos que não utilizamos pesticidas ou outras substâncias químicas, uma vez que não são essenciais para o desenvolvimento e a proteção das culturas. Além disso, graças às análises contínuas efectuadas nos campos, verificamos a qualidade e as eventuais necessidades e/ou deficiências durante a fase vegetativa. Além disso, estas análises representam a base para a amplificação dos canabinóides, o que nos permite avaliar a percentagem de ingredientes activos presentes durante o crescimento das plantas e, consequentemente, decidir qual é o momento certo para a colheita.

Colheita
A colheita é feita através de equipamento agrícola específico que corta as plantas de Cannabis Sativa L. e as desloca diretamente para um reboque ao lado, à medida que as plantas vão sendo lentamente cortadas. Durante esta fase (como em todas as fases do trabalho), damos muita importância à limpeza de todas as máquinas/equipamentos que entram em contacto com o material vegetal, de forma a evitar o risco de contaminação antes, durante e após a colheita.


Secagem
Para obter um produto que cumpra os padrões de qualidade exigidos, o processo de secagem desempenha um papel fundamental para evitar qualquer tipo de degradação, alteração ou contaminação das plantas de Cannabis Sativa L. Depois de colhido, o material vegetal é transportado (em condições secas e limpas) para locais de secagem especificamente dedicados ao processamento do cânhamo. A fase de secagem, sendo um ponto crucial do processamento do cânhamo e, consequentemente, da qualidade do produto final, é cuidadosamente monitorizada, nomeadamente através do controlo contínuo de parâmetros fundamentais como a temperatura e o tempo de secagem, a circulação do ar e a humidade relativa do secador.


O controlo destes três factores constitui uma garantia operacional para obter um produto acabado uniformemente seco, evitar a formação de bolores e a contaminação de todo o material vegetal e permitir uma conservação correcta e adequada ao longo do tempo.
Separação mecânica
Uma vez seco, o material é submetido a uma separação mecânica. Esta fase de processamento permite separar perfeitamente o material vegetal através de uma máquina que funciona automaticamente esfregando escovas e passando por peneiras vibratórias e peneiras fixas. Uma vez terminado o processo mecânico, o material residual será completamente separado do material perfeitamente moído útil para os nossos fins (extração de princípios activos) que será embalado e armazenado.
Embalagem e armazenamento

Uma vez terminado o processo de separação, o material moído passa diretamente da máquina separadora para as bocas de saída específicas, que têm nas suas extremidades sacos (especificamente destinados a uso alimentar, limpos e secos, permitindo a transpiração mas sem contacto com substâncias externas/possíveis contaminantes) para a embalagem final. O ciclo da máquina permite assim um enchimento controlado e contínuo (reduzindo a passagem e intervenção dos operadores). No entanto, salientamos o facto de que todos os materiais utilizados durante as diferentes fases de processamento estão registados e são acompanhados de fichas técnicas que atestam a sua compatibilidade e utilidade para os nossos objectivos qualitativos.
O material embalado é, portanto, armazenado em paletes. Nesta fase ocorre a etiquetagem final dos lotes homogéneos, com um número de lote individual que resume todos os dados de processamento, permitindo uma rastreabilidade clara e precisa do produto. As paletes são armazenadas em zonas específicas e bem ventiladas do armazém, com acessos protegidos, espaços de fácil limpeza e subdivididos por fase de processamento. Uma vez terminado o processo de embalagem, o material aguarda a expedição para o nosso local de produção (ou para os clientes).
PRODUÇÃO DE ÓLEO CBD
Para obter um extrato, é necessário separar os compostos interessantes da planta daqueles que não têm qualquer utilidade para nós. Mais especificamente, cada extrato Enecta é desenvolvido e formulado em torno de um canabinóide específico. Os canabinóides podem ser separados da matéria vegetal de muitas maneiras diferentes. Desenvolvemos métodos específicos para garantir a segurança e a eficácia deste processo. É por isso que podemos garantir que o melhor óleo CBD também pode ser acessível aos nossos clientes.


Orientações farmacêuticas
O cânhamo embalado e armazenado está pronto para ser enviado para o nosso local de produção (ou para os clientes), onde o material vegetal será submetido a um processamento adicional para obter o produto final. A nossa prioridade é manter elevados padrões de gestão da qualidade no desenvolvimento, produção e controlo dos nossos produtos, de forma a garantir que estes cumprem simultaneamente os requisitos de segurança, qualidade e eficiência.
Todas as fases de produção seguem os requisitos das BPF (Boas Práticas de Fabrico, regras e instruções aplicáveis a todas as fases do ciclo de produção) e baseiam-se num sistema de qualidade integrado fiável e em procedimentos precisos de gestão de riscos, para garantir que os produtos estão em conformidade com as directrizes de estabilidade do ICH (Conselho Internacional para a Harmonização dos Requisitos Técnicos dos Medicamentos para Uso Humano) e com os regulamentos de qualidade da ISO (Organização Internacional de Normalização) e que, por conseguinte, são adequados para a utilização pretendida e não representam um risco para o consumidor devido a precauções inadequadas em matéria de segurança ou qualidade.
Este objetivo de qualidade é alcançado quer através do trabalho do pessoal qualificado envolvido em todas as fases do processamento, quer através da utilização de procedimentos certificados nas instalações e espaços, com equipamento e serviços adequados.
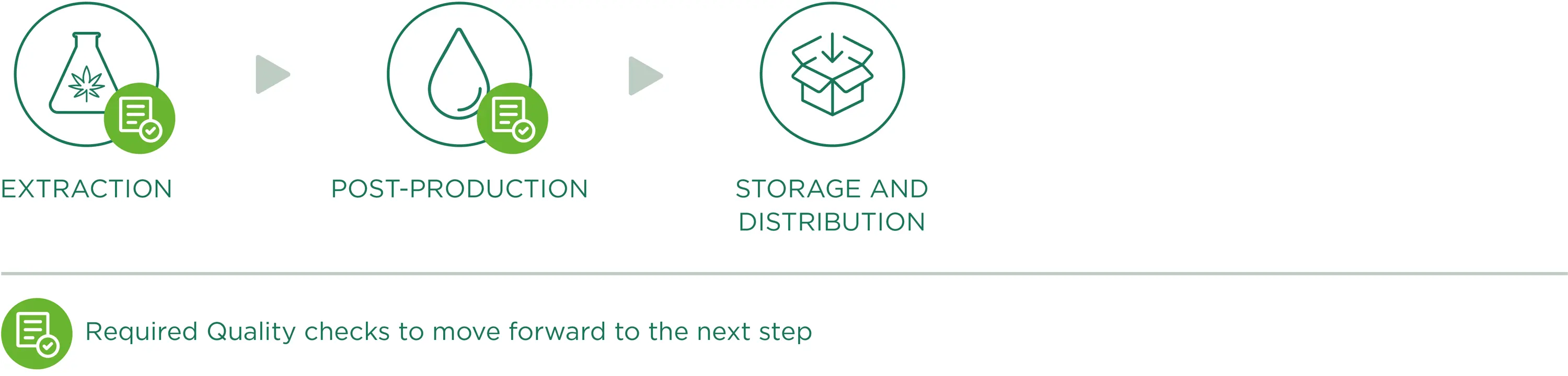
Extração de óleo CBD
Um papel fundamental da produção é a fase de extração química, que ocorre em laboratórios onde todas as operações são planeadas, verificadas, registadas e comunicadas. A extração química consiste em separar os componentes de interesse (ou seja, CBD, CBG) da matriz sólida da planta, de modo a obter apenas extractos ricos/concentrados destes componentes de interesse, com uma remoção precisa do THC. Os métodos de extração aplicados melhoram as propriedades da nossa matéria-prima, mantendo a estrutura e a atividade biológica original das suas substâncias.
As condições de extração são rigorosamente normalizadas, com especial atenção aos parâmetros do processo, incluindo a temperatura, o agente de extração e os reagentes utilizados. O nosso processo envolve a utilização de solventes GRAS (Classe III) como o EtOH; as diferentes etapas seguintes garantem a separação de interferentes (como a cera) que perturbariam as fases de purificação e isolamento de um canabinóide específico, o que deve acontecer sem degradar a sua estrutura molecular natural. Este processo estruturado permite-nos trabalhar nos componentes ácidos (ou seja, CBDA) para serem descarboxilados, se necessário, reduzindo a formação de metabolitos secundários do processo.
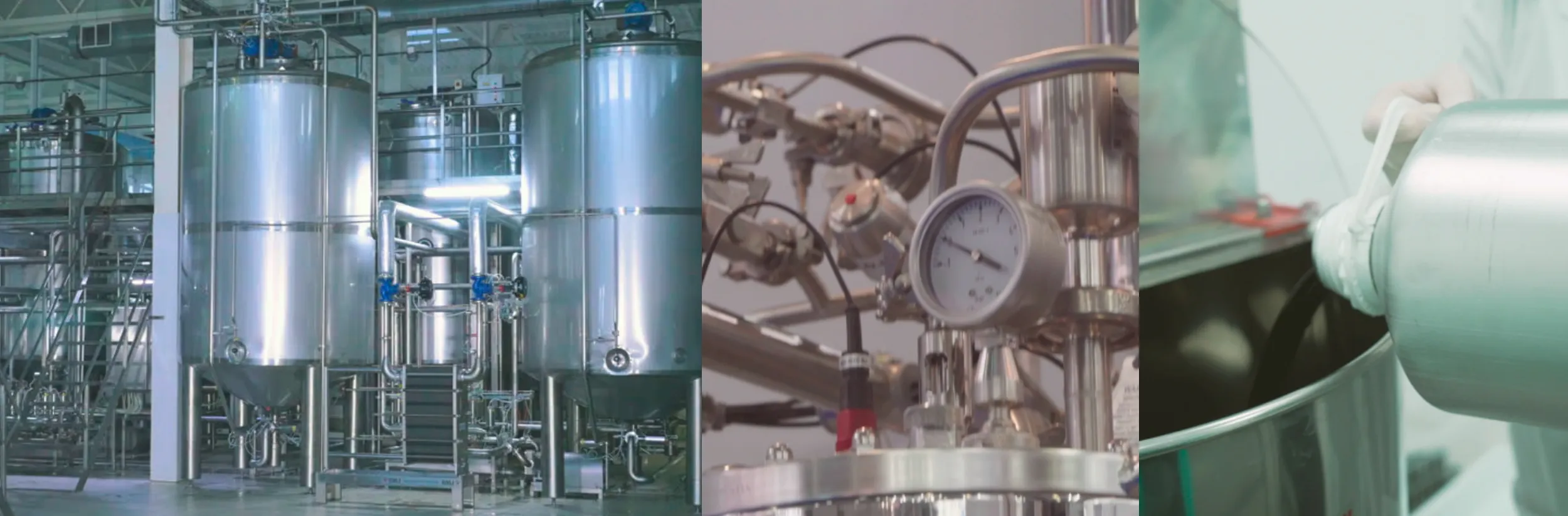
Controlo de qualidade
O controlo de qualidade aprova e implementa todos os procedimentos de controlo de qualidade: escrever os procedimentos pormenorizados para a amostragem e os testes durante e após o processo; manter a documentação e os registos de produção; definir os procedimentos de limpeza e sanitização das instalações; verificar as condições de funcionamento (calibração e manutenção) das ferramentas, máquinas e equipamentos; monitorizar a eficiência do processo; garantir a correcta rotulagem dos recipientes e a conformidade da embalagem; monitorizar a estabilidade do produto; implementar sistemas eficazes de rastreabilidade. O controlo da qualidade não se limita, portanto, às operações laboratoriais, uma vez que está envolvido em todas as decisões relativas à qualidade do produto (desde o cultivo da matéria-prima até à embalagem e distribuição do produto final).

Pós-produção
Quanto à embalagem dos produtos, esta pode ser dividida em diferentes categorias, com base na sua finalidade e no seu papel na cadeia de produção. Por "embalagem" entende-se o material de acondicionamento destinado a conter os produtos e a assegurar a sua proteção, apresentação e permitir o seu manuseamento seguro desde o produtor até ao consumidor.
Em geral, as embalagens podem ser divididas em primárias e secundárias. A primeira categoria refere-se a recipientes diretamente em contacto com o produto (ou seja, frascos, garrafas), enquanto a segunda categoria consiste na embalagem que encerra o material primário (ou seja, caixas para garrafas pequenas, etc.).
O objetivo de uma embalagem de qualidade, ou seja, o que é pedido para garantir que o consumidor é..:
- Perfeitamente adaptado ao produto, protegendo a sua integridade e impedindo qualquer penetração potencial de agentes externos;
- Resistência efectiva às condições ambientais externas (nomeadamente para as embalagens primárias, que devem ser resistentes à luz e herméticas);
- Proteção adequada do produto com características precisas para garantir a integridade do produto durante o movimento ou o transporte;
- Comunicação correcta sobre a natureza dos conteúdos.
Com efeito, os produtos podem deteriorar-se devido a incompatibilidades químicas entre os componentes da formulação ou com os materiais de embalagem, ou ainda devido aos efeitos da humidade, do oxigénio, da luz e das diferentes temperaturas. Por exemplo, a utilização de recipientes opacos (de metal, de plástico colorido ou de vidro âmbar) permite proteger o produto da luz e das reacções de oxidação. A embalagem deve conter o número de lote do produto para permitir a rastreabilidade e ter as informações necessárias para identificar o produto (nome, composição qualitativa e quantitativa, data de validade), permitindo a rastreabilidade da sua autenticidade. A rastreabilidade é um processo que segue o produto de montante a jusante na cadeia de produção e garante que, em cada fase, os vestígios (informações) adequados estão presentes e incluídos na criação do número de lote.


Armazenamento e distribuição
Os locais dedicados à armazenagem dos produtos destinam-se a: assegurar boas condições de armazenagem; permitir a armazenagem ordenada e categorizada das mercadorias e reduzir ao mínimo o risco de contaminação; de um modo geral, evitar qualquer efeito negativo na qualidade dos produtos; manter condições de armazenagem adequadas (luz, temperatura, humidade, etc.) e, por conseguinte, manter constantes os padrões de qualidade e integridade durante a armazenagem e o transporte.